CBP-Gcm interaction during gliogenesis
Subgroup Leader : Pierre CATTENOZ
Teams : Transcriptional regulation of neural and immune development
One of the most fascinating questions in modern biology is to understand how do we interact with and respond to the environment. The nervous and the immune systems are key players in the process and interact with each other. Defects in their proper formation/function/interaction are at the basis of severe human pathologies as diverse as cancer, mental retardation, neurodegeneration and autoimmune diseases.
Our goal is to understand the molecular and cellular mechanisms underlying glial and macrophage biology, using the Drosophila model to perform in vivo studies. Glial cells represent the second major population of the nervous system and differentiate from neural stem cells that also produce neurons. These cells are highly migratory and accomplish multiple functions that allow neuronal development, activity and survival. In flies, they also act like microglia, vertebrate immune cells that migrate into the developing nervous system and become the resident macrophages. Hemocytes or macrophages represent the immune cells outside the nervous system and constitute major actors of innate immunity.
The advantages of using Drosophila are multifold. The major pathways are evolutionarily conserved, making it possible to use it to model human pathologies. Moreover, Drosophila is simpler than higher organisms, with a reduced, well annotated genome, a simpler organization including the lack of adaptive immunity and publicly available and highly sophisticated genetic tools.
We identified a shared transcription factor, Glide/Gcm, that controls gliogenesis/hematopoiesis through a sophisticated molecular network that hints to epigenetic processes. Interestingly, we found that Gcm also controls the response to acute and chronic inflammatory challenges, suggesting an ancestral role of this factor in immunity. Further emphasizing the neural-immune connection, the anti-inflammatory role of Gcm is conserved in microglia, vertebrate immune cells that migrate into the developing nervous system and become the resident macrophages. Finally, we performed vivo analyses to follow collective glial migration in the whole animal and we uncovered the mechanisms controlling the process.
Taking advantage of the technological advances, we combine high throughput assays and in vivo analyses to achieve an integrative view of the cellular processes and inter-tissue interactions occurring in development and physiology. One of the most exciting findings of single cell analyses is the high heterogeneity present in each cell type. Understanding how much such heterogeneity depends on lineage vs. regulatory processes represents one of the current challenges, as this will help understand the impact of genetic as well as epigenetic cues on cell identity and function.
Finally, and connected to heterogeneity and plasticity, migratory hemocytes likely provide signaling hubs contributing to homeostasis, a role that goes well beyond immunity. Of note, their vertebrate orthologs have been recently shown to engage in poorly understood interactions with peripheral tissues and organs, in physiological and pathological conditions, making them potential therapeutic targets.
We are currently focusing on the following axes:
Subgroup Leader : Pierre CATTENOZ
Teams : Transcriptional regulation of neural and immune development
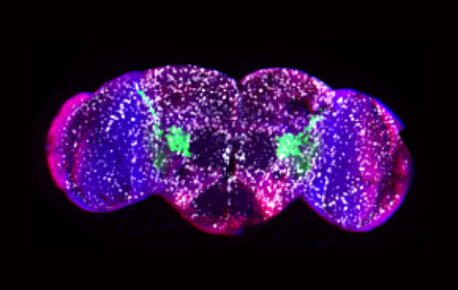
In a study published in the journal Cell Reports, IGBMC scientists discover an evolutionarily conserved anti-inflammatory transcriptional cascade from…
Read more
FEBS Journal ; Volume: 287 ; Page: 3396-3398
Cell ; Volume: 180 ; Page: 1178-1197.e20
PLoS Genetics ; Volume: 15 ; Page: e1007980
Journal of Neuroscience ; Volume: 39 ; Page: 5269-5283
Haematologica ; Volume: 104 ; Page: 717-728
Frontiers in Genetics ; Volume: 10
Journal of Neuroscience ; Volume: 39 ; Page: 238-255
Communications Biology ; Volume: 1
eLife ; Volume: 7
Disease Models & Mechanisms ; Volume: 10 ; Page: 463-474