Intrinsically disordered proteins in lipid storage and lipid transfer
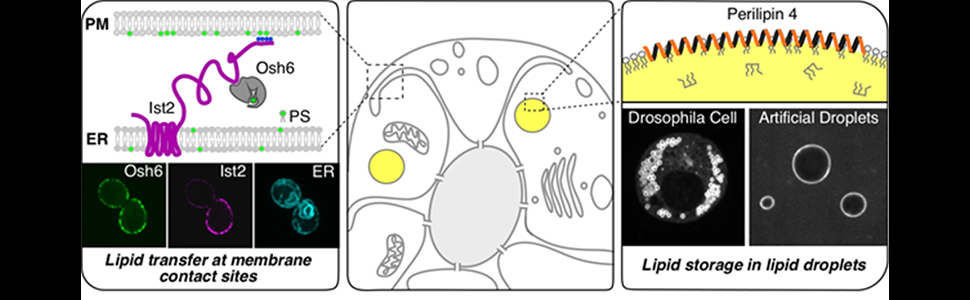
My group studies how lipids are transported within the cell and how they are stored in lipid droplets using different model systems, from yeast and cell culture to reconstituted in vitro models. I will talk about two proteins that are involved in these processes and that have been the primary focus of the work in my group. The first is a yeast protein, Ist2, which we have shown to be required for efficient transport of phosphatidylserine from the endoplasmic reticulum to the plasma membrane. The second, Plin4, is a mammalian protein that binds to lipid droplets and is highly expressed in adipocytes cells, and also in skeletal muscle. Although at a first glance, these two proteins have very little in common, they both contain an unusually long intrinsically disordered region (IDR). I will discuss how the IDRs of Ist2 and Plin4 participate in the function of these proteins. Furthermore, we recently found that the IDR of Plin4 is prone to aggregation. I will also discuss how this property may relate to muscular degeneration associated with a genetically-encoded expansion of the Plin4 IDR.
Sur invitation de
Équipe(s)
Lieu
Salle de conférence E1031, CBI
Conférencier(ère)s
Dr. Alenka COPIC
Centre de Recherche en Biologie cellulaire de Montpellier - CRBM
