Deciphering cancer cell autonomous and non-autonomous STING dependent tumor-promoting functions in KrasG12/Tp53ko mouse model of lung adenocarcinoma
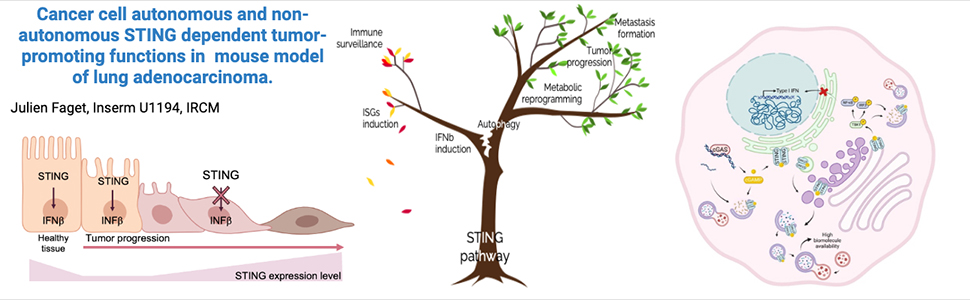
Triggering STING (Stimulator of Interferon Genes) pathway, an innate immune sensor, is considered a possible therapeutic option in lung cancer. Indeed, in tumor infiltrating myeloid cells, canonical Sting signaling, induces a potent type-I interferon (IFN) signaling, orchestrating robust immune responses. However, Sting’s role in cancer cells is complex and context dependent, being associated to tumor growth in some cancer models. Our research used the KrasLSL-G12D/WT;p53fl/fl (KP) mouse model to explore STING biology in lung cancer cells.
Strikingly, Sting depletion in cancer cells significantly impaired tumor formation in vivo and reduced metastasis to mediastinal lymph node. In vitro experiments showed that mesenchymal-like and epithelial-to-mesenchymal transition (EMT) induced cells exhibit heightened STING expression that contributes to their migration ability. The analysis of Sting signaling revealed a disruption in its canonical cascade and impairment in downstream IFN secretion. On the other hand, Sting-expressing cells show enhanced metabolic fitness and flexibility supported by Sting-dependent mTOR inhibition and autophagy. Ultimately, altered Sting function decreases cancer cell immunogenicity together with an improved metabolic fitness both, favoring disease progression and dissemination in mouse models.
Conférencier(ère)s
Dr. Julien FAGET
Immunity and Cancer Team,
Institut de Recherche en Cancérologie de Montpellier,
Inserm-U1194,
Montpellier
France
