Crosstalk between membrane-bound organelles, membrane-less condensates and the cytoskeleton in neurons
Neurons are highly polarized cells that can extend long axonal projections to connect to distant targets, which represents a challenge to ensure their homeostasis and proper activity. Microtubules ensure the long-distance transport of various cargos to distal parts of neurites. Among them, membrane-less ribonucleoprotein (RNP) granules, containing mRNAs, ribosomes and RNA-binding proteins, assemble in the same complex through liquid-liquid phase separation and are directly transported or associated with membrane-bound organelles for accurate transport along MTs. The transport of specialized axonal RNP granules and their local translation far from the cell soma allows for precise spatiotemporal and activity-dependent control of gene expression to facilitate axon and synapse development and maintenance.
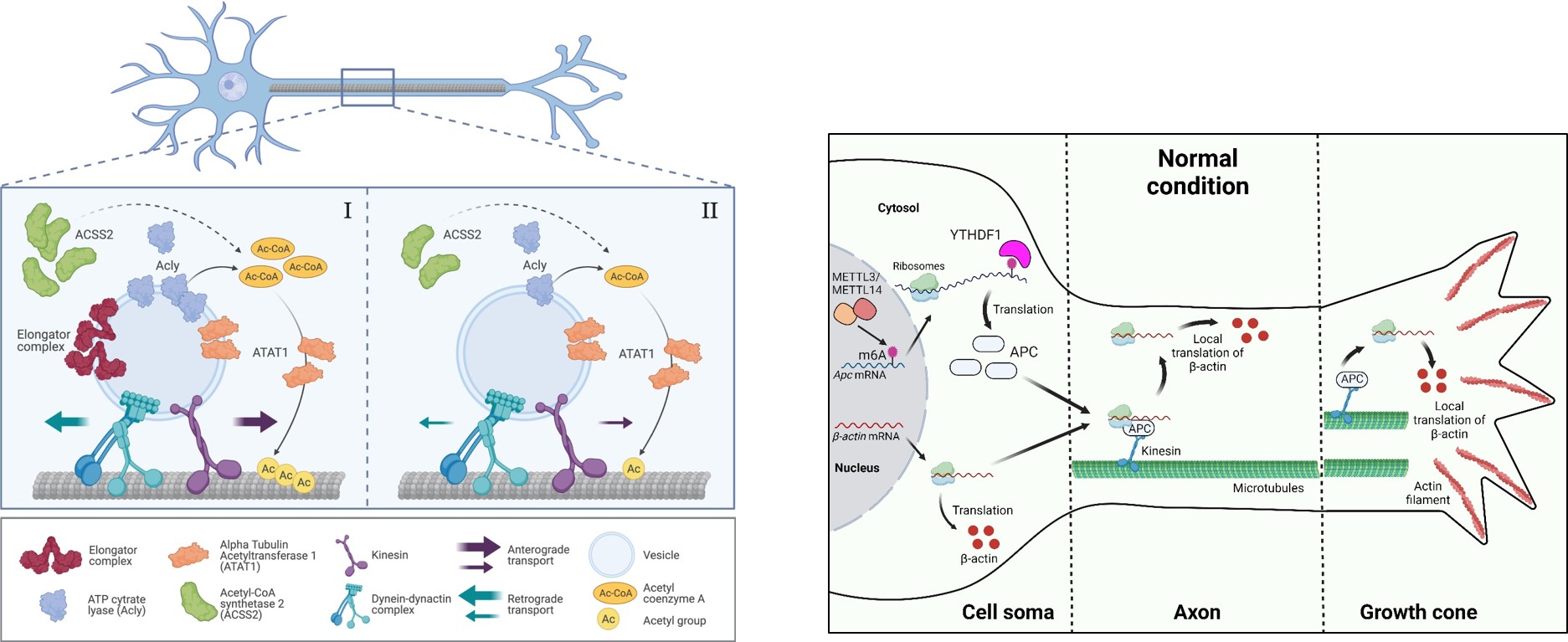
To date, no universal localization cis-acting elements have been identified among all distally-localized mRNAs which implies that there might be additional mechanisms beyond the primary sequences of the RNA to provide localization information in neurons. We present evidence of N6-methyladenosine (m6A)-mediated translational control of the RNA-binding protein, APC, influencing cytoskeletal dynamics at the growth cone. Our findings demonstrate that m6A modifications occur on Apc mRNA, facilitating its recognition by the m6A reader YTHDF1 to promote APC translation in neuronal somata. Additionally, we observe that disrupting the m6A pathway impairs the transport and local translation of β-actin mRNA in the axon and growth cones, a deficiency that can be rescued by the exogenous expression of APC protein in cultured neurons. Our findings suggest a novel mechanism involving m6A-mediated regulation of APC protein translation, linking epitranscriptomics to axonal mRNA targeting, cytoskeletal dynamics, and axon development.
Accumulating evidence suggests that defects in RNP granule assembly, transport, and translation activation are common features of various neurodegenerative diseases including amyotrophic lateral sclerosis, frontotemporal dementia and spinal muscular atrophy. Intriguingly, inter-organelle membrane contact site disruption has emerged as a pathological mechanism underlying neurodegenerative disorders. However, although inter-organelle interactions between membrane-less and membranous organelles have been identified, the function of these contacts remains largely unknown. In particular, it is not known whether the ER, the largest membranous organelle, known to control the function and dynamics of nearly every organelle through the formation of membrane contact sites, regulates RNP granule homeostasis in neurons. I will discuss how I plan to reveal the molecular and functional connectome between membrane-less RNP transport granules and the ER, how the ER orchestrates the different steps of the RNP granules’ life-cycle in healthy neurons, from the specificity of their assembly to their subcellular localization and local translation and how these processes are altered in the context of neurodegenerative diseases.
Conférencier(ère)s
Loic BROIX
RIKEN Center for Biosystems Dynamics Research
Dan Ohtan Wang’s laboratory
Japon
