Mechanisms Underlying the Transformation of Neural Progenitor Cells (NPCs) in Platelet-Derived Growth Factor A (PDGFA)
The lethal brain cancer, Glioblastoma (GBM), has a distinctive genomic architecture, but no known cause, modifiable risk factor, or curative treatment. Furthermore, our understanding of GBM is limited by our inability to appreciate how it begins by analyzing human tumor tissue and by the absence of authentic laboratory models of this cancer.
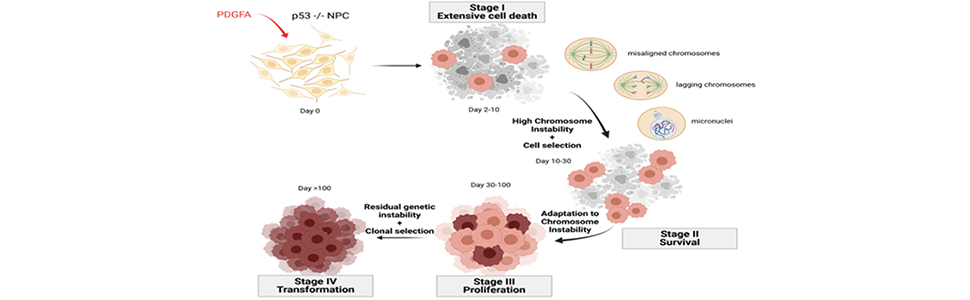
Here, we examine a mouse model of GBM developed in our laboratory to determine how exposure to Platelet-Derived Growth Factor-AA (PDGFA), a brain-abundant mitogen, transforms neural progenitor cells (NPCs), a putative GBM cell-of-origin. In our model, P53 null NPCs from the subventricular zone (SVZ) of young adult mice divide abnormally with widespread cell death. Cells that survive defective mitosis have unstable genomes and resume proliferating at an increasing rate, become tumorigenic, and acquire a GBM-like genome. Deciphering these events may shed light on the origins of GBM and identify targets for therapy.
We show that PDGFA fails to induce the transcription of FoxM1 leading toincomplete expression of the kinetochore while simultaneously activating FOS and forcing mitotic errors. Defective mitosis together with impaired mitotic surveillance, as seen in P53 null cells, allows NPCs to survive with chromosome instability (CIN). Thereafter, survivors spontaneously resume dividing, accumulating chromosomal rearrangements, a change in phenotype accompanied by over-expression and phosphorylation of EGFR, increasing expression of Jun, re-expression of FoxM1 and the kinetochore, and return of FOS to basal levels, all in the presence of PDGFA. These adaptations allow NPCs with CIN to complete mitosis, proliferate, and achieve growth factor independence, and can be prevented by knockout of Fos and/or early inhibition of EGFR.
By stimulating their proliferation without setting the stage for error-free mitosis, PDGFA transforms P53 null NPCs and generates Egfr amplified GBM-like cancer cells. These findings encourage further exploration of our model as a segue to understanding the origins of GBM.
Sur invitation de
Équipe(s)
Lieu
Salle de réunion 4004, IGBMC
Conférencier(ère)s
Mathieu MEODE
Arnie Charbonneau Cancer Institute
University of Calgary
États-Unis
