The quaternary structure of the GR highlights inter-domain allosteric communication
In inflammatory or autoimmune diseases, modulating the action of the glucocorticoid receptor (GR) is an effective therapeutic strategy. However, the adverse effects of these treatments are numerous, significant and limit prescriptions.In a study published in Nature Structural & Molecular Biology, researchers from IGBMC and AstraZeneca present the multi-domain structure of the glucocorticoid receptor. This structure provides an in-depth understanding of the molecular mechanism of GR signaling and may provide insights for the design of the next generation of drugs.
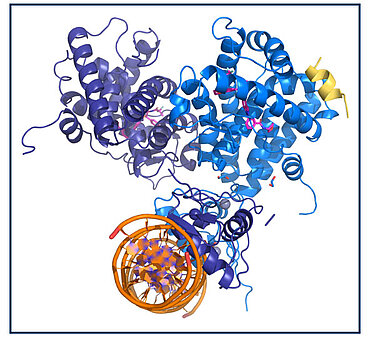
High resolution crystal structure of the glucocorticoid receptor while interacting with an agonist and bound to its DNA binding site. Crédits : Isabelle M.L. Billas, IGBMC
The glucocorticoid receptor is a ligand-activated transcription factor that binds to corticosteroid hormones. Thus, it mediates their regulatory effects on the cardiovascular, immune and musculoskeletal systems. In inflammatory and autoimmune diseases, synthetic GR agonists, such as dexamethasone and prednisone, are effective but cause undesirable side effects such as metabolic disorders, insulin resistance and/or muscle wasting.
GR is a modular protein that encompasses several regions including the ligand binding domain and the DNA binding domain. To elucidate the signaling mode of GR, it is necessary to have a molecular level understanding of each domain and how these domains communicate with each other to integrate the hormone pharmacophore with DNA sequence information.
In this collaborative study, the scientists present the multi-domain structure of GR and provide the first detailed view of a quaternary steroid receptor complex.
By exploring interface mutations in functional assays, the authors map allosteric communication pathways and shed light on the mechanisms behind disease-related receptor mutations. The scientists also provide a biophysical characterization of the effects of the non-steroidal agonist ligand velsecorat and the steroidal ligands dexamethasone and fluticasone furoate on the receptor conformation. Through this, they highlight the specific communication of the ligand across the different domains, connecting the ligand and DNA binding events.
These results provide insight into how receptor modifications can lead to disease progression and may guide future drug design.
- A. High-resolution crystal structure (2.5 Å resolution) of DNA-bound GR, activator peptide (yellow), and GR velsecorat agonist (pink) show that GR forms an asymmetric dimer on DNA. Arrows highlight cross-domain interactions and putative allosteric communication pathways.
- B. GR ligand binding induces ligand-specific structural rearrangements. Velsecorat (Vel, magenta) and fluticasone furoate (FF, dark gray) are shown as bound within the ligand binding pocket.
Crédits : Isabelle M.L. Billas, IGBMC
