When protein complex construction and protein synthesis occur simultaneously
The activity of our cells is regulated by multiprotein complexes, essential for their functioning. In a study published in Nature Structural & Molecular Biology, Dr László Tora's team investigates the molecular mechanisms of the assembly of a multiprotein complex involved in the activity of our genes. They show that this complex begins to assemble as early as the RNA-to-protein conversion stage, shedding light on a strategy that could be widespread among these complexes.
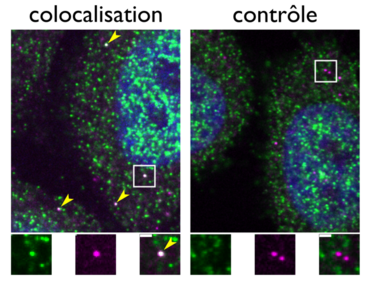
Most of the protein machineries that regulate cellular functions are made up of several, often very different, protein subunits. Efficient assembly of these protein complexes is essential to reduce non-specific interactions, protein aggregation and mislocalization within the cell. The organization of a cell can be described as a huge box of building blocks, where a single brick may encounter and interact with many other random bricks in the crowd before finding its natural partners and participating in the assembly of a complex element. The question is how cells are capable of autonomously and efficiently assembling complex protein machineries.
Many examples of these protein complexes are key factors involved in regulating gene expression in the nucleus of our cells. Among them, the general transcription factor TFIID (20 subunits) has been widely studied for its central role in driving the expression of all protein-coding genes, but where and how this complex is constructed in the cell remained an unsolved enigma.
In this study, scientists investigated the assembly steps of TFIID in human cells using a series of complementary experimental approaches. Thus, they show that most assembly steps occur before the completion of protein synthesis, in a process called co-translational assembly. The simple elements of the complex are built up when individual subunits engage in the nascent chain of their specific partners on ribosomes. These intermediates are stored in the cell's cytoplasm and participate in the final assembly step on the nascent chain of the complex's largest subunit, TAF1. The authors discovered that this subunit functions as a landing platform for protein assembly. Thus, once translation of TAF1 is complete, a complete TFIID complex is released and translocated into the nucleus.
These results show that hierarchical assembly, occurring at the same time as protein synthesis, is an effective strategy. They also enable the scientists to hypothesize that this strategy is widespread among the cell's large protein complexes.

Schematic summary of the co-translational assembly model of TFIID complex biogenesis. The diagram shows the final steps in the assembly process, which involve the association of various pre-assembled TFIID intermediates onto the nascent protein of the main subunit of the complex (TAF1) during translation. TFIID subunits are identified by numbers.
