The structure of the NuA4/Tip60 complex reveals the mechanism and importance of long-range chromatin modification
Histone acetylation facilitates gene transcription and DNA repair and is carried out by various multiprotein complexes. Among these, the NuA4 complex has the remarkable ability to acetylate its targets at a distance from its recruitment site. In an article published in Nature Structural and Molecular Biology, the scientists elucidate the structure of this complex, the way in which it acts at a distance from its binding site and the relevance of this long-range activity.
DNA, the carrier of genetic information, wraps around histone proteins to form the repeated structure of the nucleosome. Certain regions of the DNA must be accessible for gene expression or damage repair. This is why nucleosomes near these DNA sites are usually modified by acetylation, creating a decondensed form of DNA that is more accessible.
In baker's yeast, the only essential histone acetylase enzyme is incorporated into the NuA4 protein complex. Interestingly, local recruitment of NuA4 results in acetylation profiles spanning several nucleosomes at distances ten times greater than the expected size of NuA4.
NuA4 consists of four modules linked by the Eaf1 subunit, which acts as a scaffold, but little structural information is available about this complex.
Several fundamental questions remain unanswered, including how histone acetylase activity is integrated into the complex and how it targets nucleosomes located at a great distance. Other chromatin-modifying structures are also suspected of possessing long-range activity, but the mechanism and functional relevance of such activity remain elusive.
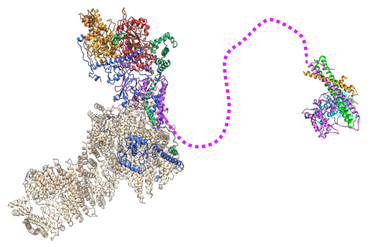
In this study, the scientists succeeded in determining the structure of NuA4 at atomic resolution. A scaffold composed of three intertwined subunits, including a protein called Ep11, connects the different modules of NuA4 and integrates the histone acetylation module. Importantly, the structure revealed that a long unstructured region of the Epl1 protein links the core of NuA4 to the acetylation module. This flexible link gives the enzyme module its long-range action relative to the core of NuA4, an essential capability for yeast proliferation, as the authors demonstrated. They also showed a reduction in H4 acetylation levels over very large genomic regions, covering several thousand base pairs, when the unstructured region of Epl1 was shortened.
This demonstrates its importance in establishing nucleosome acetylation over long distances, which is crucial for the proper functioning of yeast proliferation.

Cryo-electron microscopy structure of the core of the NuA4 complex. The acetylation module is connected by a flexible link to the Epl1 subunit.
Credit: Alexandre Frechard, IBMC
