LENOX, a melanoma-specific long non-coding RNA, regulates cell metabolism and promotes resistance to MAP Kinase inhibitors
Melanomas are composed of multiple cell types with different proliferative and invasive properties. This tumor heterogeneity represents a key feature of melanoma that complicates the development of effective treatments. In a paper published in the journal Cancer Research, Irwin Davidson's team identifies a long non-coding RNA LINC00518/LENOX expressed in all melanoma cells and necessary for their survival and tumor progression. They show that melanomas are dependent on this factor and thus highlight a new treatment target that could help overcome tumor heterogeneity.
Melanoma is the most malignant skin cancer. Despite the introduction of targeted therapies based on MAP kinase inhibitors (MAPKi) or immunotherapy, a significant fraction of patients are still considered non-responders or develop resistance. The heterogeneity of melanoma is a major obstacle to effective therapy. Many efforts have been made to understand the molecular mechanisms, both genetic and epigenetic, underlying MAPKi and immunotherapy resistance.
Melanoma tumors consist of a variety of cell types characterized by specific gene expression signatures and different proliferative, invasive, and drug-resistant properties. While naive tumors comprise a large majority of melanoma cells of the melanocytic type, different therapies result in the appearance of other cell states, including a neural crest stem cell-like state or an undifferentiated/mesenchymal state. Both of these cell states are implicated in minimal residual disease and the acquisition of treatment resistance. It is therefore important to identify targets common to all these cell states in order to eradicate melanoma cells efficiently.
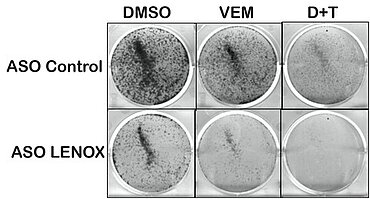
It is in this context that scientists have identified the long non-coding RNA LENOX specifically expressed in melanoma and whose expression in human melanoma is associated with poor prognosis. LENOX is essential for melanoma cell viability and tumor development. Mechanistically, LENOX interacts with the RAP2C GTPase and promotes its interaction with the mitochondrial fission regulator DRP1. This interaction stimulates the phosphorylation of DRP1 serine 637, inhibiting its function and decreasing mitochondrial fission, resulting in longer mitochondria and increased oxidative metabolism. Thus, LENOX acts to optimize mitochondrial function and cell survival capacity under stressful conditions during melanoma development and progression. In addition, LENOX facilitates the metabolic transition from glycolysis to oxidative metabolism and confers resistance to MAPKi. Therefore, inhibition of LENOX acts cooperatively with MAPKi to eradicate melanoma cells.
Thus, the scientists show in this study that melanomas are dependent on the long non-coding RNA LENOX, which optimizes mitochondrial function during melanoma development and progression. A combination of LENOX inhibition with MAPKi treatment represents a potential new therapeutic strategy to treat melanoma.
The long non-coding RNA LENOX interacts with the GTPase RAP2C allowing its interaction with DRP1. This interaction stimulates the phosphorylation of serine 637 of DRP1 inhibiting its function and decreasing the fission of mitochondria. This decrease in fission leads to an increase in oxidative metabolism (OXPHOS), survival of melanoma cells and resistance to MAPKi treatments.
