Actin dynamics and biomechanics of the early embryo
Actin dynamics and biomechanics of the early embryo
 During embryonic development, cell architectures are constantly remodelling to enable proper cellular function and cell interaction with its environment. The actin-cytoskeleton networks are the structures that shape the cell. These dynamic cellular scaffolds allow the cell to organise itself with respect to subcellular compartments, probe its environment, and sustain the required forcesnecessary for cellular activities, such as motility or cytokinesis.
During embryonic development, cell architectures are constantly remodelling to enable proper cellular function and cell interaction with its environment. The actin-cytoskeleton networks are the structures that shape the cell. These dynamic cellular scaffolds allow the cell to organise itself with respect to subcellular compartments, probe its environment, and sustain the required forcesnecessary for cellular activities, such as motility or cytokinesis.
In constant assembly, turnover or remodelling, the actin architectures are incredibly dynamic. Actin is organized in a variety of dedicated architectures, in a spatiotemporally regulated manner using hundreds of different building blocks collectively referred to as actin-binding proteins. Altering the availability or localization of particular actin-binding proteins allows the cell to tune its properties, thus affecting cell morphology, mechanics and gene expression profiles, which can then feedback into the processes controlling cell fate.
Our ambition is to reveal how the actin-cytoskeleton shapes and dynamics control cell-identity acquisition during the early Caenorhabditis elegans embryonic development. The C. elegans embryo has the unique property whereby the fates of each cell are known, thus allowing us to view the impact of perturbations with precision. To do so we combine an interdisciplinary expertise at the interface between biochemistry and cell biology together with high quality microscopy and quantitative analysis. We are studying how actin architectures are spatiotemporally controlled in the different cell types found in the early C. elegans embryo and how some specificities could impact cell commitment in the differentiation process during the process of early development and differentiation. The goal is to understand the biochemical process of actin regulation and dynamics in vivo, but also how these differ in case of known genetic perturbation as those mimicking human rare diseases, for example.
.jpg)

Members
Researchers
Post-doctoral fellows
PhD students
Technicians
Former members
Delphine Suhner, Assistant Engineer until March 2021.
Saurabh Tak, Postdoctoral fellow until October 2019.
Current projects
Project 1 – Actin dynamics and cell identity
Each embryo experiences enormous challenges in order to successfully transform a passive oocyte loaded with parental material into a polarized embryo of differentiated blastomeres. C. elegans follows a determinate model of development during which invariant cleavage patterns set up reproducible patterns of cell interactions. In addition to rapid volume reduction, asymmetries arise in daughter cells both biochemically and physically, leading in a few divisions to a reproducible but diverse collection of founder cells, the blastomeres.
By the use of in vivo endogenous fluorescent labeling of actin binding proteins (the building blocks) using CRISPR/Cas9 genetic engineering as well as state of the art live imaging techniques and quantitative image analysis, we are studying in the early embryonic cells, the dynamics of individual actin binding proteins and how differences of content or dynamics arise along the lineage.
Our goal is to reveal how actin architectures are spatiotemporally controlled in the different cell types found in the early C. elegans embryo and how some actin specificities could impact cell commitment in the differentiation process during the process of early cell identity acquisition of the blastomeres.
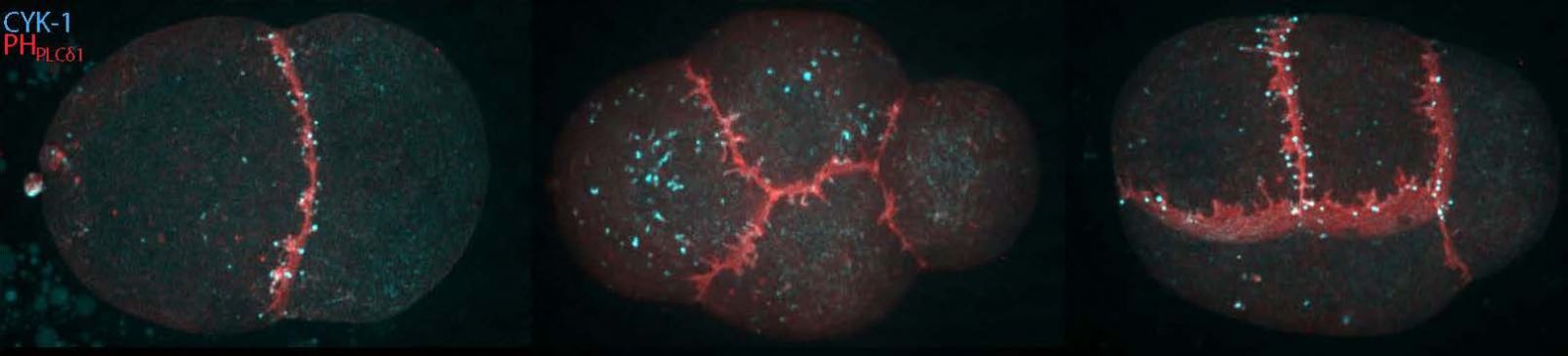
Project 2- CRISPR targeted mutagenesis of C. elegans actin: novel insights into the understanding of human non-muscle actinopathies.
Human mutations in the cytoplasmic actin genes (ACTB and ACTG1) cause a broad spectrum of rare disorders named Non-Muscle Actinopathies (NMAs). NMAs show high clinical variability and results often in pleotropic neurodevelopmental disorders, including the Baraitser-Winter cerebro-fronto-facial syndrome (BWCFFT).
The five research groups of the European EJP RD PredACTINg consortium have combined their expertise to explore NMA disease mechanisms at a multi-scale level, ranging from single molecules to model organism. The project aims at identifing genotype to phenotype correlations and thus in the future explain the clinical consequences of NMAs resulting from ACTB and ACTG1 mutations. The Reymann team is reproducing some actinopathies related human mutations, in the model organism C. elegans using CRISPR/Cas9 mediated genome engineering. These mutants will be used to assess the developmental defects with particular focus on neural development, as well as the perturbation of actin organization and actin dynamics in the early embryonic cells using our fluorescently labelled strains. We aim at uncovering the developmental and cellular consequences of identified human genetic mutations in cytoplasmic actin genes
The long-term goal of study is to allow a substantial revision of treatment management strategies, provide the basis for future clinical trials and yield broadly applicable functional assays that facilitate genotype-phenotype correlation.
EJP RD PredACTINg consortium: Dr. Med. Di Donato (Faculty of Medicine Carl Gustav Carus, TU Dresden, Germany), Prof. Dr. Manstein (Medizinische Hochschule Hannover, Institut für Biophysikalische Chemie, Hannover, Germany), Prof. Dr. Kellermayer (Department of Biophysics and Radiation Biology, Semmelweis University, Budapest, Hungary), Dr. Bianco (PhysioLab, Department of Biology, University of Florence, Italy) and Dr. Reymann.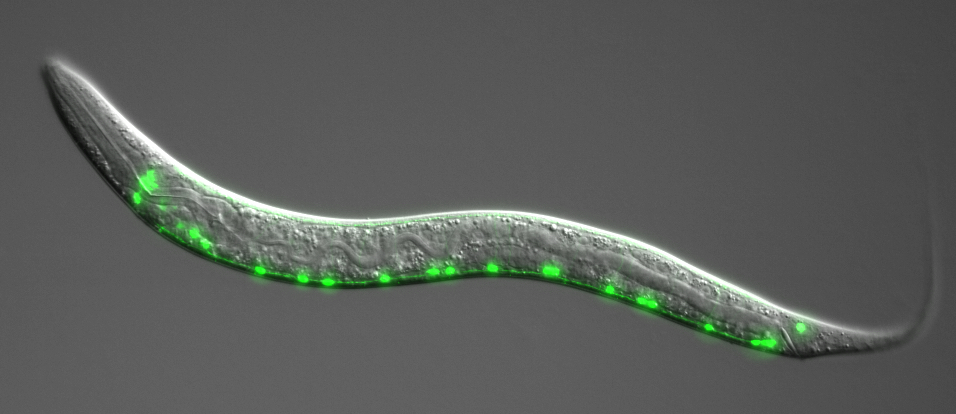
Collaborations and networks
Nataliya Di Donato, TU Dresden, Dresden, Germany.
Nate Goehring, The Francis Crick Institute, London, United Kingdom.
Francois Robin, Institut de Biologie Paris Seine, Paris, France.
GDR Approches Quantitatives du Vivant
Société Française de Biologie du Développement
Genie, Group of Elegans New Investigators in Europe
Funding and partners
- 2020-2023 EJP RD, Predacting, European Consortium, coordinator Med Dr N. Di Donato.
- 2019-2023 ANR JCJC, DeCaNu.
- 2018-2019 IDEX Grant, University of Strasbourg.
- 2017-2019 LABEX Start-Up Package of the IGBMC, CERBM GIE.

Publications
2025
Article in a journal
Multiscale characterization of Caenorhabditis elegans mutants to probe functional mechanisms of human actin pathological variants
- Théo Hecquet
- Nadine Arbogast
- Delphine Suhner
- Anaïs Goetz
- Grégory Amann
- Selin Yürekli
- Fiona Marangoni
- Sophie Quintin
- Johannes N Greve
- Nataliya Di Donato
- Anne-Cécile Reymann
iScience ; Volume: 28 ; Page: 113652
Article in a journal
In vivo detection of ALFA-tagged proteins in C. elegans with a transgenic fluorescent nanobody
- Sophie Quintin
- Maria Izabella Saad
- Grégory Amann
- Anne-Cécile Reymann
microPublication biology
2024
Article in a journal » Review article
Acto-myosin clusters as active units shaping living matter
- Karsten Kruse
- Rémi Berthoz
- Luca Barberi
- Anne-Cécile Reymann
- Daniel Riveline
Current Biology ; Volume: 34 ; Page: R1045-R1058
Pre-publication, Working Document
Classification of human actin pathological variants using C. elegans CRISPR-generated models
- Théo Hecquet
- Nadine Arbogast
- Delphine Suhner
- Anaïs Goetz
- Grégory Amann
- Selin Yürekli
- Fiona Marangoni
- Johannes N Greve
- Nataliya Di Donato
- Anne-Cécile Reymann
2023
Article in a journal
Meeting report: Third Franco‐Japanese developmental biology meeting “New Frontiers in developmental biology: Celebrating the diversity of life”
- Masayuki Oginuma
- Anne-Cécile Reymann
Genesis - The Journal of Genetics and Development ; Volume: 61
Article in a journal
The kinesin Kif21b regulates radial migration of cortical projection neurons through a non-canonical function on actin cytoskeleton
- José Rivera Alvarez
- Laure Asselin
- Peggy Tilly
- Roxane Benoit
- Claire Batisse
- Ludovic Richert
- Julien Batisse
- Bastien Morlet
- Florian Levet
- Noémie Schwaller
- Yves Mély
- M. Ruff
- Anne-Cécile Reymann
- Juliette Godin
Cell Reports ; Volume: 42 ; Page: 112744
2022
Article in a journal
Rapid assembly of a polar network architecture drives efficient actomyosin contractility
- Vlad Costache
- Serena Prigent Garcia
- Camille N Plancke
- Jing Li
- Simon Begnaud
- Shashi Kumar Suman
- Anne-Cécile Reymann
- Taeyoon Kim
- François B Robin
Cell Reports ; Volume: 39 ; Page: 110868
2019
Article in a journal
Anterior-enriched filopodia create the appearance of asymmetric membrane microdomains in polarizing C. elegans zygotes
- Nisha Hirani
- Rukshala Illukkumbura
- Tom Bland
- Greǵoire Mathonnet
- Delphine Suhner
- Anne-Cécile Reymann
- Nathan W Goehring
Journal of Cell Science
Awards and recognitions
2015 AAAS Newcomb Cleveland Prize, for my participation to the Chen et al, Science, 2014 publication.
Thesis Prize 2012 from the "Fondation Nanosciences" and Thesis Prize 2012 of the PRES "University of Grenoble" for my PhD work.

