Differentiation and physiopathology of endocrine cells in the pancreas and intestine
Differentiation and physiopathology of endocrine cells in the pancreas and intestine
Our main goal is to decipher the transcriptional networks controlling the differentiation, function and maintenance of endocrine cells in the pancreas and intestine in normal and pathological conditions.
Future strategies for cell replacement therapies and regenerative medicine strongly depend on our knowledge of the detailed mechanisms that control the differentiation of multipotent stem cells into highly specialized cells. Along these lines of research, our goal is to understand how the diversity of pancreatic and intestinal endocrine cells is generated from stem/progenitor cells during embryogenesis towards adult life.
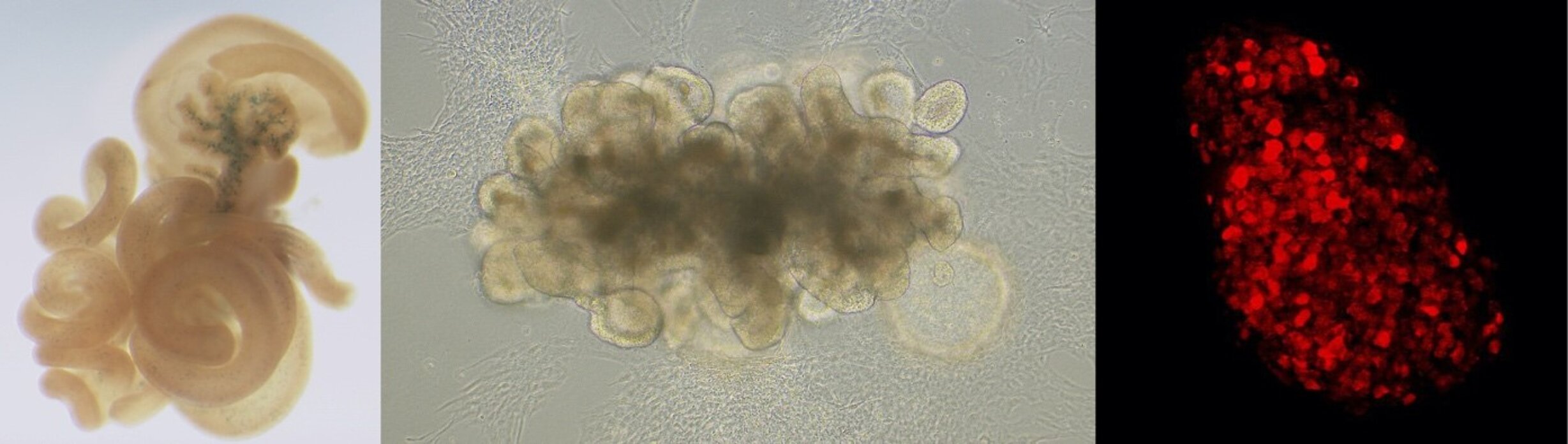
Pancreatic endocrine cells are clustered in islets embedded in the exocrine tissue. Islets contain five hormone producing cell-types, including insulin-secreting beta cells, which, in concert, control glucose homeostasis. Absolute or relative insulin-deficiency lead to diabetes. Intestinal endocrine cells, also called enteroendocrine cells (EECs), are very closely related to pancreatic islet cells with respect to their embryonic origin, differentiation programs and physiological roles in the control of energy homeostasis. EECs are rare cells found along the intestinal mucosa, they sense nutrients in the gut lumen and, in response, secrete a variety of hormones that act locally or at distance to regulate energy homeostasis via their control of intestinal absorption, food intake and insulin secretion. We showed previously that pancreatic and intestinal endocrine cells arise from progenitor cells expressing the pro-endocrine transcription factor Neurog3. In absence of Neurog3, islet cells and EECs do not form leading to neonatal diabetes and intestinal malabsorption in mice and human. Despite the identification of a few targets of Neurog3, the molecular mechanisms implementing Neurog3 endocrinogenic function are poorly understood.
We focus on the identification and study of novel effectors controlling endocrine sub-type specification and functional maturation. To tackle these questions, we use stem cell based human organoid culture systems and mouse models combined with gene editing and multi-omics approaches. We hope that our studies will contribute to the development of a cell-based therapy in diabetes, as well as to understand the mechanisms underlying the pathophysiology of islet and enteroendocrine hormone failures in human.

Members
Researchers
PhD students
Technicians
Funding and partners
The team was supported by founding from
- Novonordisk Foundation
- Agence National pour la Recherche (ANR)
- Fondation pour la Recherche Médicale (FRM)
- Novonordisk
- National Institute for Diabetes and Digestive and Kidney Disease (NIDDK)
- Société Francophone du Diabète (SFD)
- Juvenile Diabetes Research Foundation (JDRF)
- European Union (6th FP)
- Institut Benjamin Delessert
- Association Française des Diabétiques (AFD)
- Université de Strasbourg
- ARC
- INSERM-AVENIR
News
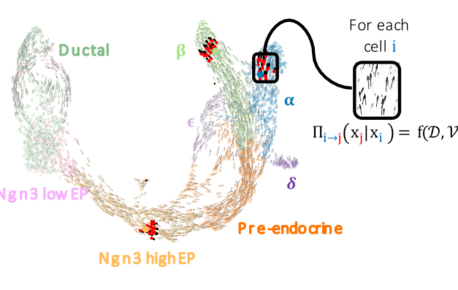
FateCompass: to identify and predict the decisive criteria of cell fate
Cell differentiation is a process regulated by gene expression through the action of transcription factors. Different transcription factors and…
Read more
Resources
Human intestinal organoid. Enteroendocrine progenitors are expressing the transcription factor NEUROG3 (red).
https://seafile.igbmc.fr/f/80de9329f382470d80de/?dl=1

