Common Mechanisms of Development, Cancer and Aging
Common Mechanisms of Development, Cancer and Aging
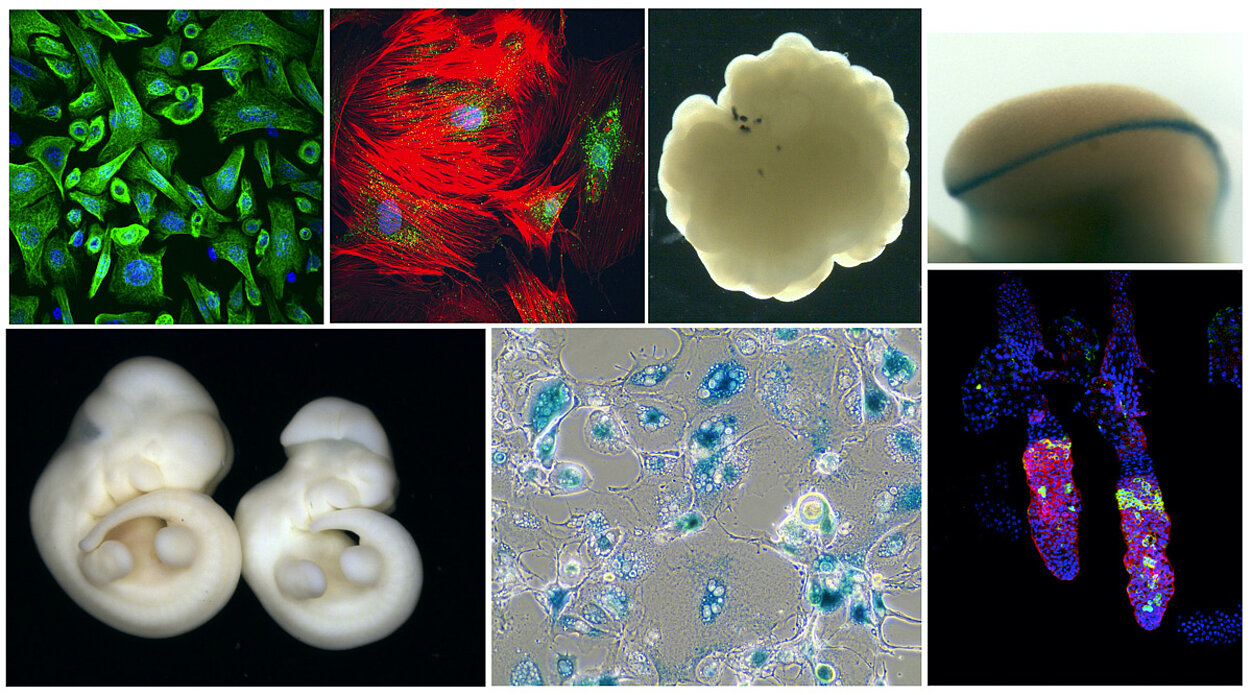
Cellular Senescence
Cellular Senescence is a state of irreversible cell cycle arrest induced in response to a variety of stimuli, including tissue and cellular damage, inflammatory signals and developmental cues. However, despite being arrested from the cell cycle, senescent cells remain highly active, and interact with their environment through a complex secretome known as the senescence-associated secretory phenotype (SASP). The research in our lab is focused on the study of cellular senescence.
A primary function of senescence is to prevent proliferation of damaged cells, and it therefore acts as a tumor-suppressive mechanism that protects from cancer. Furthermore, the abnormal or chronic accumulation of senescent cells contributes to the aging process, and to many disease states. However, work from our group and others has identified that in certain situations, senescent cells can have beneficial functions, including during embryonic development and in tissue regeneration. We are therefore interested to understand the biological functions of cellular senescence in both physiological and pathological states, its mechanisms of action, and the consequences of its misregulation.
Members
Researchers
Post-doctoral fellows
Former members
Ph.D. Students (year graduated and last known position)
- Matteo Pecoraro (2014: Research Assistant, Institute for Research in Biomedicine, Bellinzona, Switzerland)
- Valeria Di Giacomo (2014: Scientific Writing Manager, ThePaperMill, Barcelona)
- Mekayla Storer (2014: Group Leader, Wellcome-MRC Cambridge Stem Cell Institute)
- Birgit Ritschka (2017: Postdoc, Elly Tanaka Lab, IMBA Vienna,)
Postdocs (years in the lab and last known position)
- Jason Doles (2011-2013: Assistant Professor, Mayo Clinic College of Medicine and Science, Rochester, Minnesota)
- Mari Carmen Ortells (2013-2017: R&D Senior Scientist, Biokit, Barcelona)
- Daniel Amaya (2016-2017: Postdoc, Max Planck Research Unit for Neurogenetics, Frankfurt)
Current projects
What is the role of cellular senescence during development?
We previously identified that programmed cellular senescence is a normal feature of embryonic development (Storer et al, 2013). We are investigating the functions, and molecular regulation of developmental senescence in a variety of models.
Is aberrant senescence linked to developmental birth defects?
Aberrantly induced or chronic senescence is detrimental, contributing to the aging process and multiple diseases. However, it is not clear if mis-timed or ectopic senescence contributes to developmental defects and disorders. We are investigating whether alterations in senescence may contribute to developmental disorders (e.g. Rhinn et al, BioRxiv, 2021).
How does transient senescence contribute to cell plasticity and tissue regeneration?
We have shown that transient exposure to the SASP can induce cellular plasticity and stem cell fate. We demonstrated this in skin, showing how keratinocytes exposed to the SASP become functional hair-follicle stem cells (Ritschka et al, 2017). We are exploring how the SASP can instruct such changes in cell fate, and the functional consequences of this interaction in both physiological and pathological settings, such as cancer.
How does mis-timed senescence contribute to disease, such as cancer or neurodegeneration?
The aberrant induction of senescence contributes to aging and disease. We recently identified how senescence-like changes may develop in a heterogenous manner and block tissue regeneration (Ritschka et al, 2020). We are investigating how senescence contributes to diseases such as cancer and neurodegeneration.
Can we identify novel cellular features or bio-markers of cellular senescence?
Senescent cells are very heterogenous and are difficult to identify in vivo. We are performing cell-based studies to identify novel markers and features of the dynamic senescence program.
Funding and partners
The team has been generously supported by funding from:
- Fondation Recherche Medicale (FRM)
- Fondation ARC pour la Recherche sur le Cancer (ARC)
- Worldwide Cancer Research
- La Ligue Contre le Cancer
- IDEX, University of Strasbourg
- Fonds National de la Recherche (FNR), Luxembourg
- Agence Nationale de la Recherche (ANR)
- Fondation Schlumberger
News

A projet supported by La Fondation Recherche Alzheimer
What if certain "ageing" cells, known as senescent cells, play a key role in Alzheimer's disease?
Read more
Awards and recognitions
- 2018 Academy of Sciences Cancer Research Prize from the Simone and Cino Del Duca Foundation
- 2018 Schlumberger Foundation award for research and education (Laureate)
- 2016 INSERM Research Director (Level DR2)
- 2013 The City of Barcelona Prize for Life Sciences
Publications
2023
Article in a journal
Cellular senescence and developmental defects
- Annabelle Klein
- Muriel Rhinn
- William M Keyes
FEBS Journal ; Volume: 290 ; Page: 1303-1313
2022
Article in a journal
Senescence diversity in muscle aging
- Matej Durik
- William M Keyes
Nature Aging ; Volume: 2 ; Page: 570-572
Article in a journal
Aberrant induction of p19Arf-mediated cellular senescence contributes to neurodevelopmental defectshiers.
- Muriel Rhinn
- Irene Zapata-Bodalo
- Annabelle Klein
- Jean-Luc Plassat
- Tania Knauer-Meyer
- William M Keyes
PLoS Biology ; Volume: 20
Article in a journal
Endothelial cells give a boost to senescence surveillance
- Daniel Sampaio Gonçalves
- William M Keyes
Genes and Development ; Volume: 36 ; Page: 511-513
Article in a journal
Cell cycle gene regulation dynamics revealed by RNA velocity and deep-learning
- Andrea Riba
- Attila Oravecz
- Matej Durik
- Sara Jiménez
- Violaine Alunni
- Marie Cerciat
- Matthieu Jung
- Céline Keime
- William M Keyes
- Nacho Molina
Nature Communications ; Volume: 13 ; Page: 2865
2020
Article in a journal
The senotherapeutic drug ABT-737 disrupts aberrant p21 expression to restore liver regeneration in adult mice
- Birgit Ritschka
- Tania Knauer-Meyer
- Daniel Sampaio Gonçalves
- Alba Mas Malavila
- Jean-Luc Plassat
- Matej Durik
- Hugues Jacobs
- Elisa Pedone
- Umberto Di Vicino
- Maria Pia Cosma
- William M Keyes
Genes and Development ; Volume: 34 ; Page: 489-494
2019
Article in a journal
Cellular senescence in development, regeneration and disease
- Muriel Rhinn
- Birgit Ritschka
- William M Keyes
Development (Cambridge, England) ; Volume: 146 ; Page: dev151837
2017
Chapter of the book
Detection of Senescence Markers During Mammalian Embryonic Development
- Mekayla Storer
- William Keyes
Oncogene-Induced Senescence ; Volume: 1534 ; Page: 199-210
Article in a journal
DeltaNp63alpha promotes adhesion of metastatic prostate cancer cells to the bone through regulation of CD82
- V Di Giacomo
- T Tian
- Alba Mas Malavila
- M Pecoraro
- L Battle-Morera
- L Noya
- J Martín-Caballero
- J Ruberte
- William M Keyes
Oncogene ; Volume: 36 ; Page: 4381-4392
Article in a journal
The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration
- Birgit Ritschka
- Mekayla Storer
- Alba Mas
- Florian Heinzmann
- Mari Carmen Ortells
- Jennifer Morton
- Owen Sansom
- Lars Zender
- William M Keyes
Genes and Development ; Volume: 31 ; Page: 172-183

