Differentiation and physiopathology of endocrine cells in the pancreas and intestine
Differentiation and physiopathology of endocrine cells in the pancreas and intestine
Our main goal is to decipher the transcriptional networks controlling the differentiation, function and maintenance of endocrine cells in the pancreas and intestine in normal and pathological conditions.
Future strategies for cell replacement therapies and regenerative medicine strongly depend on our knowledge of the detailed mechanisms that control the differentiation of multipotent stem cells into highly specialized cells. Along these lines of research, our goal is to understand how the diversity of pancreatic and intestinal endocrine cells is generated from stem/progenitor cells during embryogenesis towards adult life.
Pancreatic endocrine cells are clustered in islets embedded in the exocrine tissue. Islets contain five hormone producing cell-types, including insulin-secreting beta cells, which, in concert, control glucose homeostasis. Absolute or relative insulin-deficiency lead to diabetes. Intestinal endocrine cells, also called enteroendocrine cells (EECs), are very closely related to pancreatic islet cells with respect to their embryonic origin, differentiation programs and physiological roles in the control of energy homeostasis. EECs are rare cells found along the intestinal mucosa, they sense nutrients in the gut lumen and, in response, secrete a variety of hormones that act locally or at distance to regulate energy homeostasis via their control of intestinal absorption, food intake and insulin secretion. We showed previously that pancreatic and intestinal endocrine cells arise from progenitor cells expressing the pro-endocrine transcription factor Neurog3. In absence of Neurog3, islet cells and EECs do not form leading to neonatal diabetes and intestinal malabsorption in mice and human. Despite the identification of a few targets of Neurog3, the molecular mechanisms implementing Neurog3 endocrinogenic function are poorly understood.
We focus on the identification and study of novel effectors controlling endocrine sub-type specification and functional maturation. To tackle these questions, we use stem cell based human organoid culture systems and mouse models combined with gene editing and multi-omics approaches. We hope that our studies will contribute to the development of a cell-based therapy in diabetes, as well as to understand the mechanisms underlying the pathophysiology of islet and enteroendocrine hormone failures in human.
.png)
Members
Researchers
PhD students
Technicians
Funding and partners
The team was supported by founding from
- Novonordisk Foundation
- Agence National pour la Recherche (ANR)
- Fondation pour la Recherche Médicale (FRM)
- Novonordisk
- National Institute for Diabetes and Digestive and Kidney Disease (NIDDK)
- Société Française des Diabétiques (SFD)
- Juvenile Diabetes Research Foundation (JDRF)
- European Union (6th FP)
- Institut Benjamin Delessert
- Association Française des Diabétiques (AFD)
- Université de Strasbourg
- ARC
- INSERM-AVENIR
News
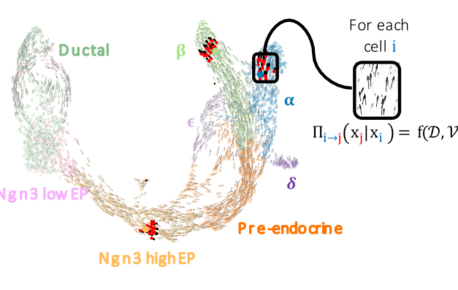
FateCompass: to identify and predict the decisive criteria of cell fate
Cell differentiation is a process regulated by gene expression through the action of transcription factors. Different transcription factors and…
Read more
Resources
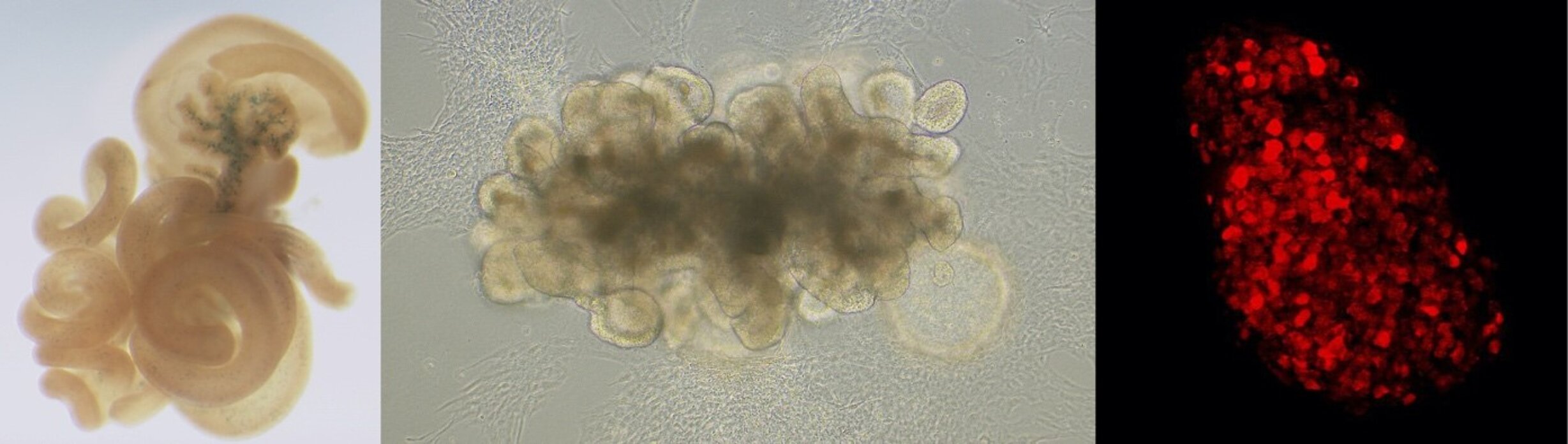
Human intestinal organoid. Enteroendocrine progenitors are expressing the transcription factor NEUROG3 (red).
https://seafile.igbmc.fr/f/80de9329f382470d80de/?dl=1
Publications
-
2011
-
Differential requirements for neurogenin 3 in the development of POMC and NPY neurons in the hypothalamus
- Michelle Pelling
- Neal Anthwal
- David Mcnay
- Gerard Gradwohl
- Andrew Leiter
- Francois Guillemot
- Siew-Lan Ang
Developmental Biology ; Volume: 349 ; Page: 406-416
-
-
2010
-
Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development
- Josselin Soyer
- Lydie Flasse
- Wolfgang Raffelsberger
- Anthony Beucher
- Christophe Orvain
- Bernard Peers
- Philippe Ravassard
- Julien Vermot
- Marianne L. Voz
- Georg Mellitzer
- Gerard Gradwohl
Development: For advances in developmental biology and stem cells ; Volume: 137 ; Page: 203-212
-
Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival
- Georg Mellitzer
- Anthony Beucher
- Viviane Lobstein
- Pascal Michel
- Sylvie Robine
- Michèle Kedinger
- Gérard Gradwohl
Journal of Clinical Investigation ; Volume: 120 ; Page: 1708-1721
-
Expression of neuropeptide Y and agouti-related peptide in the hypothalamic arcuate nucleus of newborn neurogenin3 null mutant mice
- Yuta Arai
- Gerard Gradwohl
- Yoko Kameda
Cell and Tissue Research ; Volume: 340 ; Page: 137-145
-
-
2008
-
Transcription factor PROX1 induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype.
- Tatiana V Petrova
- Antti Nykänen
- Camilla Norrmén
- Konstantin I Ivanov
- Leif C Andersson
- Caj Haglund
- Pauli Puolakkainen
- Frank Wempe
- Harald von Melchner
- Gérard Gradwohl
- Sakari Vanharanta
- Lauri A Aaltonen
- Juha Saharinen
- Massimiliano Gentile
- Alan Clarke
- Jussi Taipale
- Guillermo Oliver
- Kari Alitalo
Cancer Cell ; Volume: 13 ; Page: 407-19
-
Characterization of the proneural gene regulatory network during mouse telencephalon development
- Julia M. Gohlke
- Olivier Armant
- Frederick M. Parham
- Marjolein V. Smith
- Celine Zimmer
- Diogo S. Castro
- Laurent Nguyen
- Joel S. Parker
- Gerard Gradwohl
- Christopher J. Portier
- François Guillemot
BMC Biology ; Volume: 6
-
GeneSpeed Beta Cell: an online genomics data repository and analysis resource tailored for the islet cell biologist
- Nayeem Quayum
- Alecksandr Kutchma
- Suparna A. Sarkar
- Kirstine Juhl
- Gerard Gradwohl
- Georg Mellitzer
- John C. Hutton
- Jan Jensen
Experimental Diabetes Research ; Volume: 2008
-
-
2007
-
Temporal Control of Neurogenin3 Activity in Pancreas Progenitors Reveals Competence Windows for the Generation of Different Endocrine Cell Types
- Kerstin Johansson
- Umut Dursun
- Nathalie Jordan
- Guoqiang Gu
- Friedrich Beermann
- Gérard Gradwohl
- Anne Grapin-Botton
Developmental Cell ; Volume: 12 ; Page: 457-465
-
-
2005
-
Genetic determinants of pancreatic epsilon-cell development.
- R Scott Heller
- Marjorie Jenny
- Patrick Collombat
- Ahmed Mansouri
- Catherine Tomasetto
- Ole D Madsen
- Georg Mellitzer
- Gerard Gradwohl
- Palle Serup
Developmental Biology ; Volume: 286 ; Page: 217-24
-
Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice.
- Mercè Martín
- Jabier Gallego-Llamas
- Vanessa Ribes
- Michèle Kedinger
- Karen Niederreither
- Pierre Chambon
- Pascal Dollé
- Gérard Gradwohl
Developmental Biology ; Volume: 284 ; Page: 399-411
-
Page 3 of 4

